We are happy to offer your Organization the ability of using the BCW Serology Panel to broaden quality control and training programs, and be part of an international database where Participant Organizations measure their performance statistically.
This independent, private Serology Proficiency Panel includes a combination of any or all of the following markers:

Each BCW Serology Panel contains ten vials, regardless of marker configuration. Each vial contains at least 1.5mL of frozen plasma. The BCW Serology Panel can be configured in one of the following five options:
| P7 PANEL | P61 PANEL | P62 PANEL | P4 PANEL | P3 PANEL | |
|---|---|---|---|---|---|
| Chagas | |||||
| HIV | |||||
| HBsAg | |||||
| HCV | |||||
| HTLV | |||||
| HBc | |||||
| RPR |
Each BCW Serology Panel will be assigned a unique Panel ID that will be used for online results reporting and comparison with other panels that have the same configuration. Similarly, each vial has a unique ID that will be used for marker results reporting and comparison with all other vials that have been tested for the same marker, regardless if they make up or not panels with the same configuration.
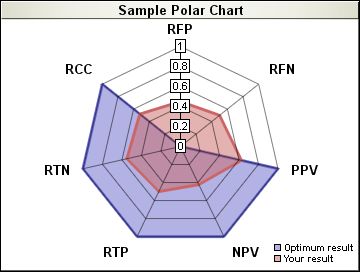
| Rate of Correctly Classified (RCC) | Number of true positives plus number of true negatives divided by the total number of samples. |
| Rate of False Positives (RFP) | Number of false positives divided by the total number of negatives (1-Specificity). |
| Rate of False Negatives (RFN) | Number of false negatives divided by the total number of positives. |
| Positive Predictive Value (PPV) | Number of true positives divided by the total number of positives. |
| Negative Predictive Value (NPV) | Number of true negatives divided by the total number of negatives. |
| Rate of True Positives (RTP) | Number of true positives divided by the total number of positives registered (Sensitivity). |
| Rate of True Negatives (RTN) | Number of true negatives divided by the total number of negatives registered (Specificity). |
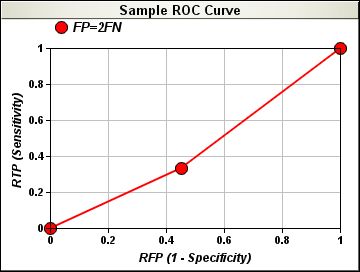
Taking two of the above indicators (sensitivity against the measure of 1-specificity) the BCW Serology Panel presents the Receiver Operating Characteristic curve (ROC curve) for each vial, marker and the aggregate panel.
The area under the ROC curve (AUR), minimum of 0.5 and maximum of 1, will be the value calculated immediately for all panels and markers right after the results are reported online. The graphic presentation of the corresponding ROC curves will show area shapes and values that will allow comparisons to be taken on a relative basis; therefore comparisons are valid for panels with the same configuration or different configuration as well as by marker. Additionally, Participants will be asked to categorize their institutions at the moment of ordering their BCW Serology Panels, therefore comparisons will be available for peer groups as follows:
The BCW Serology Panel has the following individual prices:
| P7 PANEL | P61 PANEL | P62 PANEL | P4 PANEL | P3 PANEL |
|---|---|---|---|---|
| US$1,500.00 | US$1,400.00 | US$1,300.00 | US$800.00 | US$700.00 |
Please note that shipping and handling charges are not included in the price.
Shipping costs will vary depending on address destination, and for International orders do not include any Customs duties or levies that may apply in your country. Please read the Shipping Section.
We will appreciate your payment before your order is processed for shipment. You may use Visa, Mastercard or American Express credit cards to pay your order in your Dashboard; please note that the credit card charge will be posted in U.S. Dollars.
If you prefer you may also send your payment in U.S. Dollars via bank wire transfer. A third option for payment is with a pre-approved Purchase Order sent to us via e-mail/fax.
Shipping to any U.S. address in the 50 states and District of Columbia will be made Mondays through Thursdays using FedEx guaranteed next day morning delivery; shipments will not be made on holidays.
Additional delivery time may be necessary for U.S. addresses outside the 50 states and the District of Columbia.
International shipping is available under the following general conditions: